free printable periodic table with names charges free printable
In a polyatomic ion, the group of covalently bonded atoms carries a net charge because the total number of electrons in the molecule is not equal to the total number of protons in the molecule. When drawing Lewis dot structures, the overall charge on a polyatomic ion is equal to the sum of the formal charges on each atom in the ion.
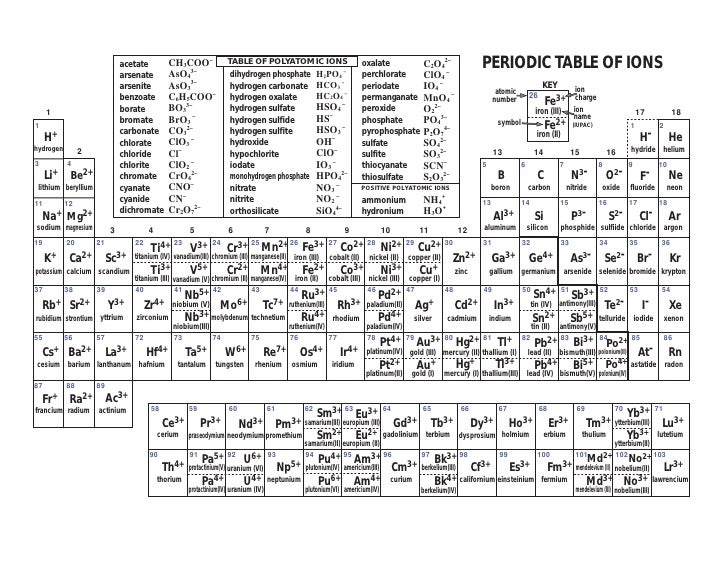
Periodic Table With Polyatomic Ions On The Back Periodic Table Timeline
3.5: Polyatomic Ions. The compound NaOH has wide industrial use and is the active ingredient in drain cleaners. Based on the discussion in the previous section, we would expect NaOH to be an ionic compound because it contains sodium, a Group 1A metal. Hydrogen and oxygen, however, are nonmetals, and we would expect these to bond together.

Polyatomic Ion Periodic Table trick (Inners and Outers) YouTube
You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas.

Chart Of Common Polyatomic Ions Bunch Riewhicur
3.2 Ions and the Periodic Table. The elements on the right side of the periodic table, nonmetals, gain the electrons necessary to reach the stable electron configuration of the nearest noble gas.. Table 3.1 Common Polyatomic Ions. Polyatomic ions can be thought of in a very similar way to monoatomic ions, in that they are ionized by either.

How to Memorize Polyatomic Ions & Chemical Formulas SuperHuman Academy
Polyatomic ions. Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. The examples include cations like ammonium ion ( NH+4 NH 4 + ), and hydronium ion ( H3O+ H 3 O + ); and anions like hydroxide ion ( OH− OH − ), and.

Memorizing polyatomic ions? Using Periodic Table Chemistry Stack Exchange
Ion Name Common Polyatomic Ions (Alphabetical order by ion name) NOTE: -ite ending means one less oxygen than the -ate form. Ion Name Acetate Ammonium Arsenate Arsenite Azide Borate Bromate Bromite Carbonate Chlorate Chlorite Ion Symbol CH3CO2- or CH3COO- NH4 + AsO4 3- AsO33- N3 - BO3 2- BrO3 - BrO2 - CO3 2- ClO3- ClO2- Ion Name Dichromate

Periodic Table Of Ions Printable Printable Word Searches
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure 5.3.2). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on.
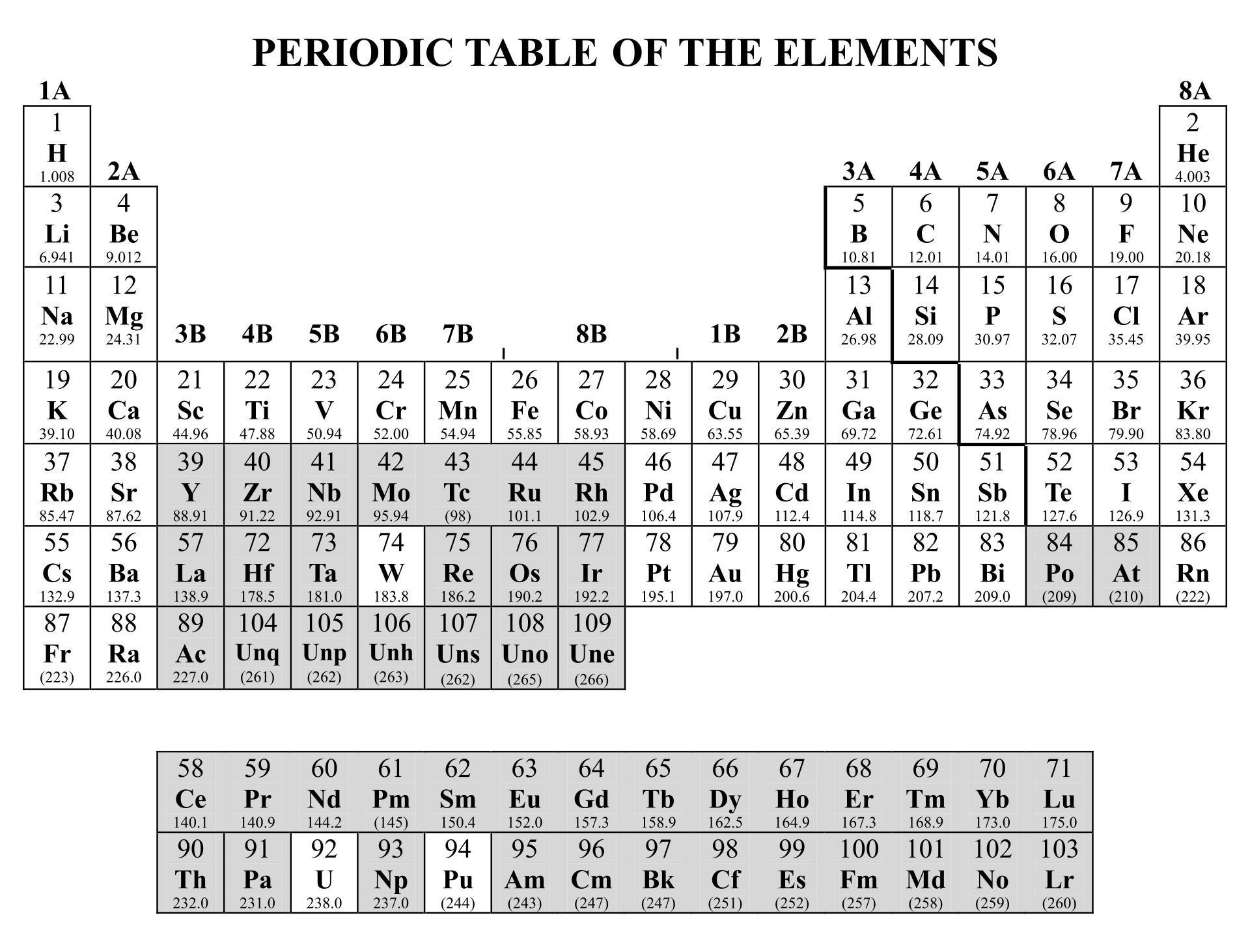
Periodic Table With Charges And Polyatomic Ions Review Home Decor
Memorizing polyatomic ions? Using Periodic Table - Chemistry Stack Exchange Memorizing polyatomic ions? Using Periodic Table Ask Question Asked 8 years, 2 months ago Modified 4 years, 7 months ago Viewed 21k times 4 In my Chemistry course, we must memorize a list of common polyatomic ions.

Free Printable Periodic Table (With names, charges & Valence Electrons
Polyatomic ions are ions that contain more than one element. This polyatomic ions list contains many common ions, grouped by charge. Each entry contains the ion's name, molecular formula and chemical structure. +1 Polyatomic Ions -1 Polyatomic Ions -2 Polyatomic Ions -3 Polyatomic Ions

Chem Naming Ionic Compounds with Polyatomic Ions Part 1 Scientific
What is a polyatomic element? The is made up of two or more atoms, it can be referred to as a polyatomic ion or a molecular ion. Depending on the charge it may be classified as cations and anions Q3 Is phosphate a polyatomic ion? Phosphate (PO 43-) is a polyatomic ion carrying a negative charge. The phosphate ion made more than one atom. Q4

Polyatomic Ions Table Free Download
IIA. Table of Polyatomic Ions. acetate CH 3COO- dichromate Cr 2O 2-. 7 dihydrogen phosphate H 2PO -. 4. ammonium NH4 + cyanide CN- silicate 2- SiO3 benzoate C6H5COO- hydroxide OH- sulphate SO4 2-. borate BO3 3- iodate - IO3 sulphite 2- SO3 carbonate 2- CO3 nitrate - NO3 hydrogen sulphide HS-.

Periodic Table With List Of Polyatomic Ions Periodic Table Timeline
As you can see from the partial table shown above the Groups of the periodic table each form a unique charge of ion. The Natural formation of ions is: Group 1 elements form +1 ions. Group 2 elements form +2 ions. Group 13 elements form +3 ions. Group 15 elements form -3 ions. Group 16 elements form -2 ions. Group 17 elements form -1 ions.
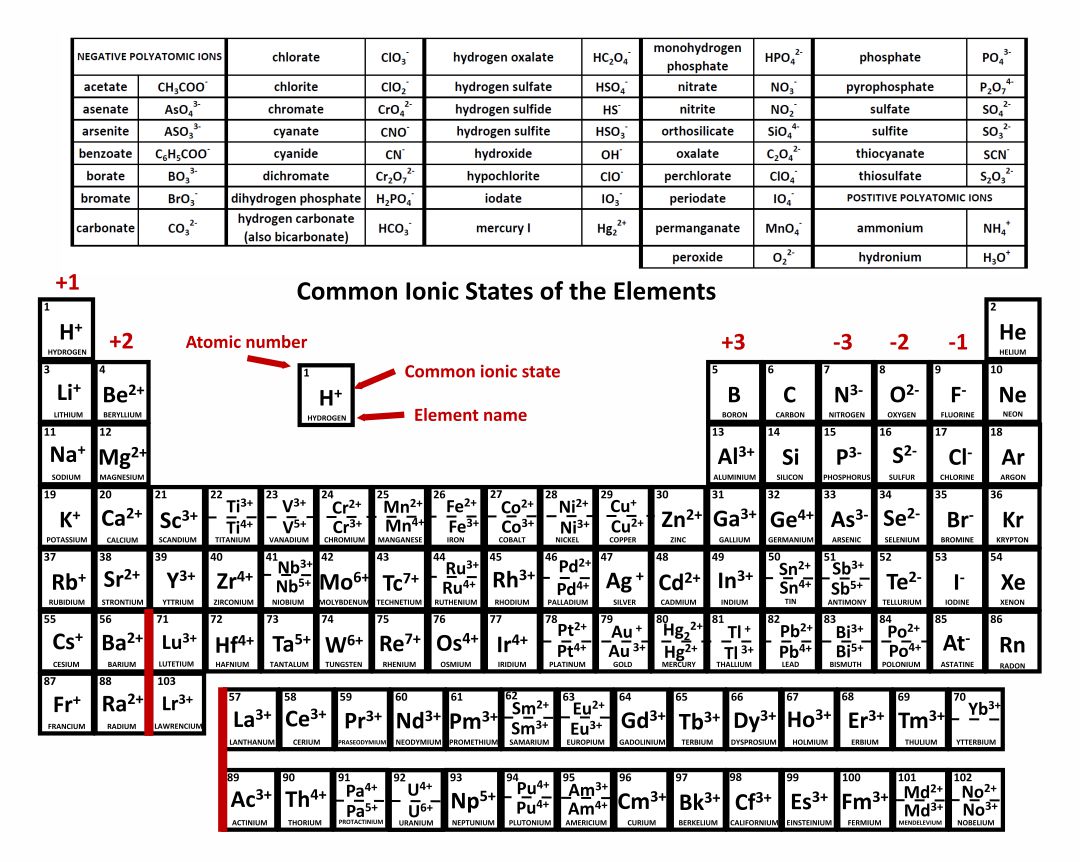
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Figure \(\PageIndex{3}\) shows.
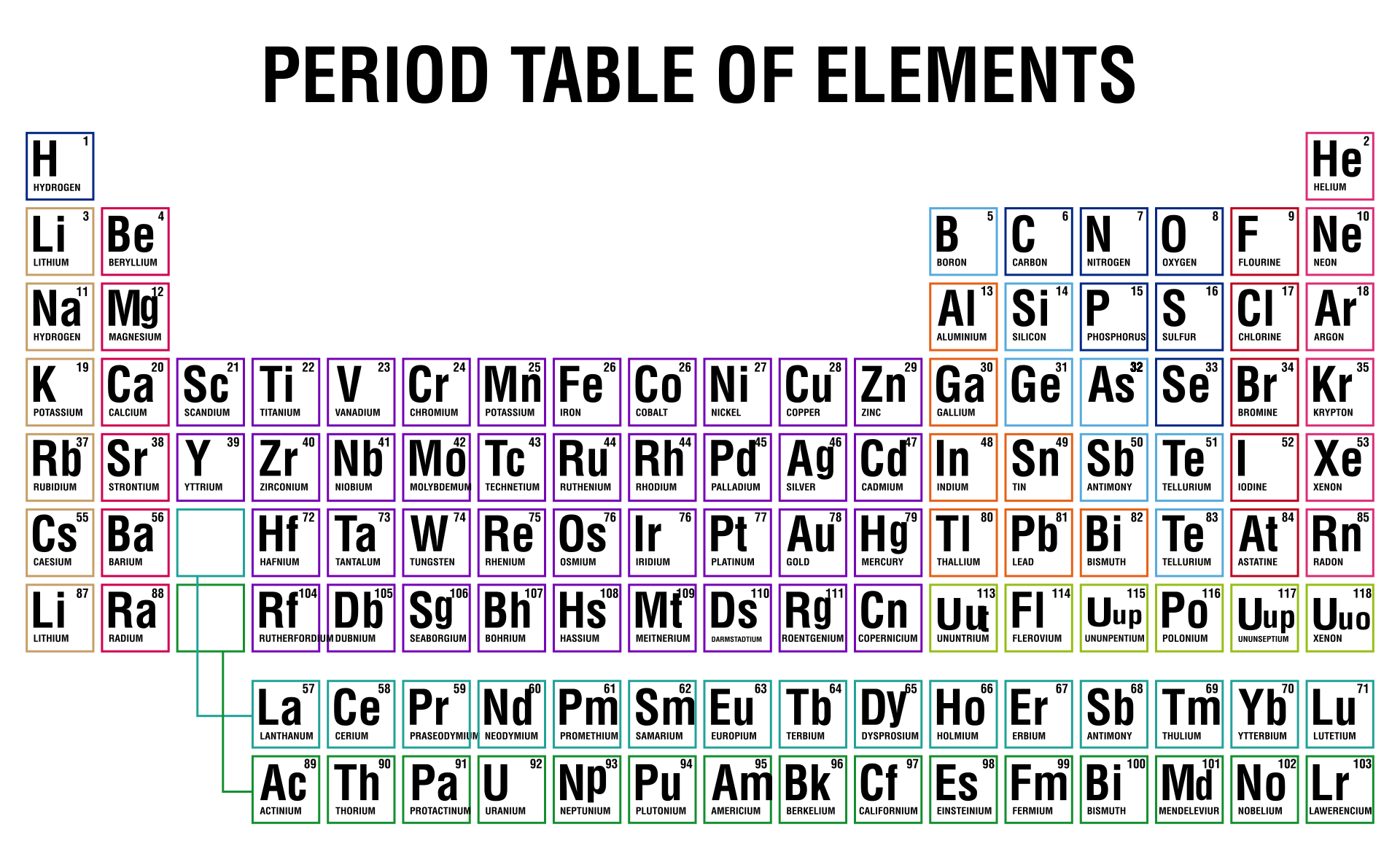
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
acetate PERIODIC TABLE OF IONS arsenate arsenite benzoate borate bromate carbonate chlorate chlorite chromate cyanate cyanide dichromate CH3COO- AsO4 3- AsO3 3- C6H5COO - BO3 3- BrO3 - CO3 2- ClO3 - ClO2 - CrO4 2- CNO- CN- Cr2O7 2- oxalate perchlorate periodate permanganate peroxide phosphate pyrophosphate sulfate.
Study Polyatomic Ions and learn how to name them
Science Intro to polyatomic ions Google Classroom Learn what polyatomic ions are and how they bond. Some ions consist of a single atom with a net charge. They're called monatomic ions. Examples include Na + , O 2 − , and Cl − . Other ions consist of a molecule —a group of atoms covalently bonded together—with a net charge.

Periodic Table With List Of Polyatomic Ions Periodic Table Timeline
Table of Polyatomic Ions. There are a number of ions that are not individual atoms but are composed of multiple atoms that are covalently bonded together. However, this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. These polyatomic ions are extremely common in chemistry and thus.